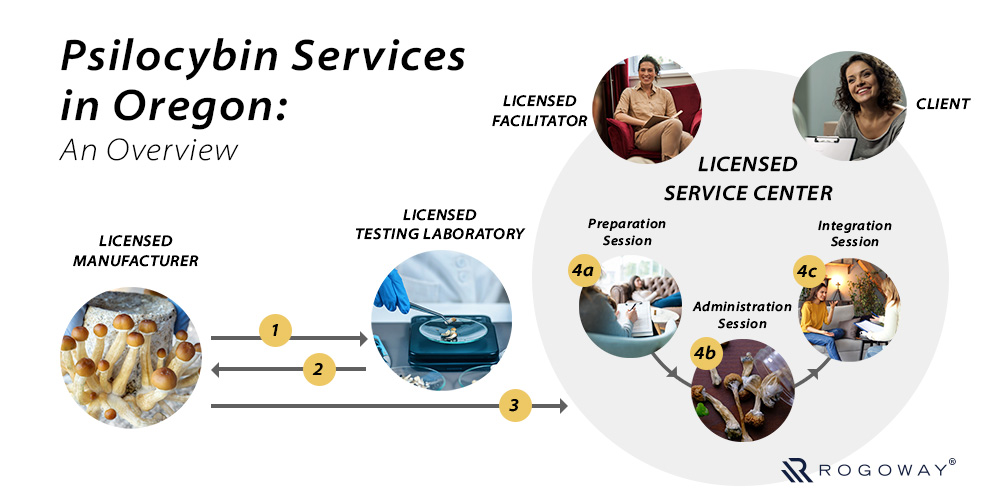
In addition to the stringent packaging requirements, Oregon Psilocybin Services also requires manufacturers to include a Product Information Document when they move any psilocybin product to licensed service centers. The Product Information Document contains manufacturer details, product details, laboratory test results, and other crucial information, which facilitators share with clients during the preparation session.
When the Product Information Document must be Provided
Per the proposed* Section 1 of Rule 333-333-2410 (Product Information Document), licensed psilocybin manufacturers in Oregon must include a product information document with all psilocybin products transferred to an authorized psilocybin service center.
As provided in proposed* Section 2 of Rule 333-333-2410 (Product Information Document), during a client’s preparation session, a psilocybin facilitator must provide the client a product information document for all psilocybin products that are planned for consumption during the client’s psilocybin administration session. Clients should have an opportunity to discuss the document with the facilitator during the preparation session.
What Should be Included in the Product Information Document
Manufacturer Details
- The manufacturer’s business or trade name and license number.
- The business or trade name of the manufacturer that packaged the product, if the manufacturer that packaged the product differs from the original manufacturer.
Product Details
In addition to the manufacturer details, the Product Information Document also contains essential details about the psilocybin product.
General Product Details
- One of the following product types:
- whole fungi
- homogenized fungi
- psilocybin extract
- edible psilocybin product.
- Species of fungi. It is important to note that per proposed* Rule 333-333-2015 (Allowable Species), licensed psilocybin manufacturers are only allowed to cultivate a single species of fungi: Psilocybe cubensis.
- Net quantity of contents using the metric system of measurement. For psilocybin extracts and other liquid products, the quantity should be expressed in terms of fluid measure (milliliters or liters). However, if the psilocybin product is solid, semi-solid, or viscous, the quantity should be listed in terms of weight (milligrams, grams, or kilograms).
- Estimated activation time, expressed in minutes.
Ingredients & Allergens
- List of all ingredients in descending order of predominance by weight or volume.
- List of potential major food allergens by:
- Listing the name of the food source of any major food allergen at the end of or immediately adjacent to the ingredient list; or
- Placing the term for the appropriate major food allergen in parenthesis within the ingredient list after the common or usual name of the ingredient derived from that major food allergen.
Important Dates
Manufacturers must include the following dates, as applicable, in the Product Information Document:
- Harvest date for whole fungi.
- Date of manufacture for homogenized fungi, psilocybin extracts, and edible psilocybin products.
- “Best by” date indicating the manufacturer-determined time that the product will retain its original quality.
Disclaimers & Warnings
If the psilocybin product is perishable, manufacturers must include a statement that the product must be refrigerated or kept frozen in the Product Information Document.
Unique Identification Number
Psilocybin manufacturers must assign a Unique Identification Number to every process lot and harvest lot per adopted Rule 333-333-2020 (General Manufacturer Requirements). This Unique Identification Number is entered into the Psilocybin Tracking System, allowing Oregon Psilocybin Services to track the product through its entire lifecycle.
A psilocybin manufacturer must include the Unique Identification Number of the psilocybin product that is being transferred to the service center in the Product Information Document.
Laboratory Test Results
Per adopted Rule 333-333-7040 (Potency Testing), psilocybin manufacturers must order tests for every batch of finished psilocybin product from a harvest lot or process lot. Laboratory testing determines the concentration (potency) of psilocybin and psilocin in the manufacturer’s product and must be included in the Product Information Document.
Technical Specifications and Accessibility
The Product Information Document can be either a printed or an electronic document as long as it meets the following requirements:
Font Size
The printed or electronic document font size must be 12-point (≈ 4.233 mm) or larger.
Language
The Product Information Document should provide all the necessary information in English.
If during a preparation session, a client requests a product information document in a language other than English, then the psilocybin service center must make reasonable efforts to translate the Product Information Document to the language, other than English, requested by the client.
Conclusion: Keep Compliance Top of Mind For Long-Term Success
Building and growing a business with compliance in mind is critical to the long-term health and success of the business. If you have questions about how the proposed* psilocybin rules will affect your psilocybin license application and business, please contact us. The psychedelic law attorneys at Rogoway Law are ready to help you with your Oregon psilocybin service center, manufacturer, and testing laboratory queries and concerns.
____________
* This post is based on rules still in draft form. The final regulations, in their entirety, are expected to be adopted by December 30, 2022. We will update this post with any pertinent information as the rules are finalized and adopted.



